
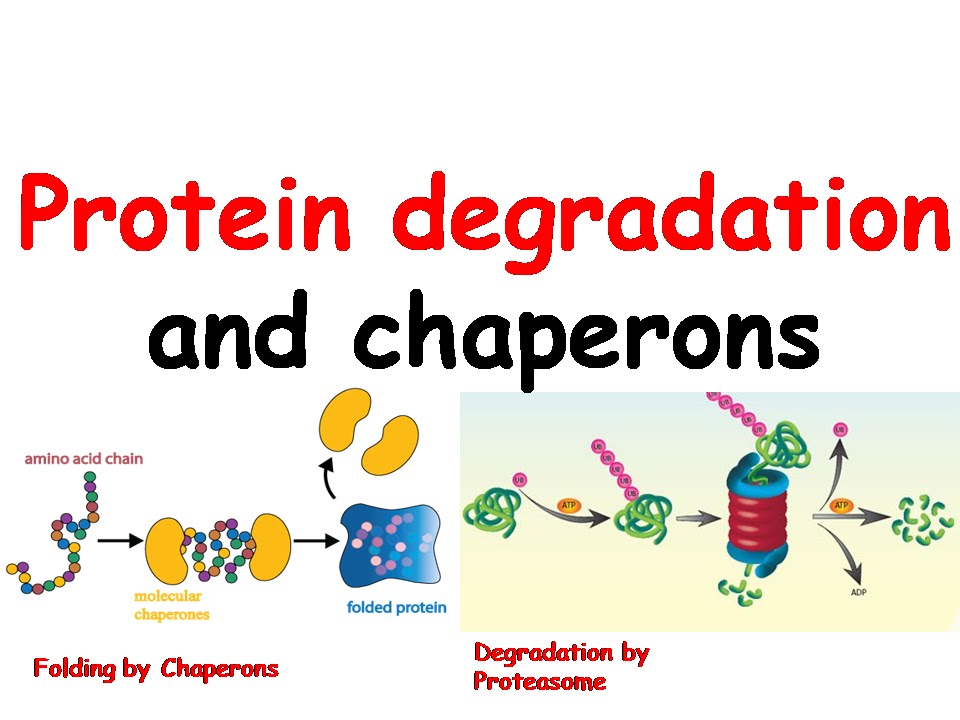
A landmark feature of molecular chaperones is the involvement of energy-dependent reactions in the folding process. The underlying functional principles of the different chaperone classes are beginning to be understood. They share the ability to recognize and bind nonnative proteins thus preventing unspecific aggregation. Link to an example.Chaperones are a functionally related group of proteins assisting protein folding in the cell under physiological and stress conditions. There are some cases where a protein can exist in more than one conformation that is, a given primary structure can give rise to two or more different tertiary structures. So the function of each of the thousands of proteins in an organism is specified by one or more genes. Perhaps some day ways will be found to treat these diseases by increasing the efficiency of disaggregating chaperones.ĭespite the importance of chaperones, the rule still holds: the final shape of a protein is determined by only one thing: the precise sequence of amino acids in the protein.Īnd the sequence of amino acids in every protein is dictated by the sequence of nucleotides in the gene encoding that protein. Protein aggregation is the cause of disorders such as Alzheimer's disease, Huntington's disease, and prion diseases (e.g., "mad-cow" disease). Not only do molecular chaperones assist in the folding of newly-synthesized proteins, but some of them can also unfold aggregated proteins and then refold the protein properly. For this reason, these chaperones are also called heat-shock proteins (HSPs). The inner wall of the cylinder is lined with hydrophobic amino acids which stabilize the hydrophobic regions of the polypeptide chain while it folds safely away from theĬhaperonins also use ATP as the energy source to drive the folding process.Īs mentioned above, high temperatures can denature proteins, and when a cell is exposed to high temperatures, several types of molecular chaperones swing into action. Some proteins are so complex that a subset of molecular chaperones - called chaperonins - is needed.Ĭhaperonins are hollow cylinders into which the newly-synthesized protein fits while it folds. The chaperones use the energy of ATP to do this work. To avoid this problem, the cells of all organisms contain molecular chaperones that stabilize newly-formed polypeptides while they fold into their proper structure. Ideally, these should be their own, but there is the danger that they could associate with nearby proteins instead - leading to aggregation and a failure to form the proper tertiary structure.


The function of a protein is determined by its shape.Rules of Protein Structure The Rules of Protein Structure


 0 kommentar(er)
0 kommentar(er)
